The superior strength and light weight of high-performance carbon and plastic composite materials have reduced the fuel consumption and harmful emissions of automobiles and airplanes and improved the efficiency of electric cars and wind turbine blades. But those same superior qualities make these indispensable materials difficult to recycle.
To avoid their disposal in landfills or recycling in high-energy, low-value industrial processes, scientists are working on ways to manufacture carbon-fiber reinforced plastic materials that allow for their triggered deconstruction and the recovery of high-quality carbon at the end of their lifespan.
But this promising alternative still creates a waste stream of material with little value, so researchers at the University of Illinois Urbana-Champaign are exploring an innovative method using a renewable energy source to upcycle this leftover material.
Chemistry professor Jeffrey S. Moore and postdoctoral researchers Yuting Zhou and Zhenchuang Xu report in a paper a new method that uses electricity to modify this recycling byproduct in a way that converts it to useful material.
The paper, “Dual C–H functionalization of polyolefins for covalent adaptable network formation via cooperative electrolysis,” is published in Nature Synthesis.
“This closes a critical loop in the life cycle of carbon fiber composites, which are widely used in wind energy, transportation, and aerospace,” Moore said.
The research team’s important modification of this byproduct happens at the molecular level through electrolysis, a process using electric current to drive a chemical reaction in a solution.
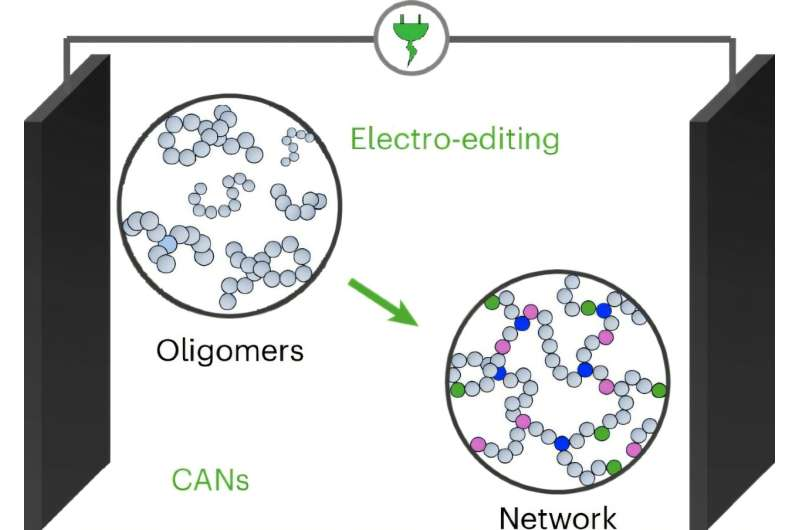
The recycling byproducts are basically fragments of the original material, the carbon-fiber reinforced polymer composite (CFRP). The fragments consist of molecules known as oligomers, smaller chains of repeating monomer units. By comparison, thermoplastic consists of linear polymers, long chains with many repeating monomer units, and CFRPs consist of crosslinked polymer matrices and carbon fiber, or networks of polymer chains that reinforce the carbon fibers.
In deconstructing the CFRPs to recover the valuable carbon fibers, the polymer matrix is broken and leaves behind the waste fragments consisting of oligomers with low mechanical properties, Zhou explained.
But through electrolysis, the researchers directly modify the oligomer backbone—the main structure of the polymer chain—at two different locations. In a single step, they modify the chain by installing two key functional groups at two carbon-hydrogen bond sites, which enables the modified oligomers to assemble into a new network formation.
“Those installed functional groups can link together, so we can link the fragments into a network. The goal is to link them [oligomers] back into a network and then restore the mechanical performance,” Zhou explained.
In this new dynamic network form, known as Covalently Adaptable Networks (CANS), this polymer material again has strong mechanical properties and the ability to be reprocessed.